- Gamefowl Eggs And Chicks For Sale
- Vaccination Program For Gamefowl Chicks For Sale
- Gamefowl Chicks For Sale Oklahoma
- Vaccination Program For Gamefowl In Philippines
- Gamefowl Roosters For Sale Chicks
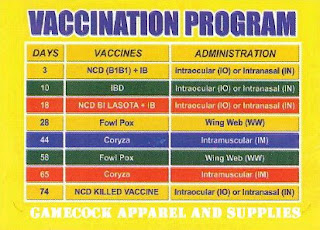
An effective vaccination programme depends on the type of operation, maternal antibody levels of day old chicks, disease challenge on the farm, age at slaughter, level of hygiene on the farm and many other factors. Apr 05, 2013 7 kind of vaccination program of Aquarius Gamefarm by Mr. Red Farm's Gamefowl Chick and Egg Management. Poultry/chicken LAsota /newcastle vaccination. With this information, I guess you can now start in gamefowl breeding. Of course, be sure to provide your birds with the best nutrition and health care available. A comprehensive vaccination program is a must considering that game birds are very susceptible to a wide range of avian pests and poultry diseases. Vaccination is an effective means to prevent and/or reduce. Florida A & M University Cooperative Extension Program. Vaccination program for a day old chicks. This is the right vaccination program for me. Gamefowl Classifieds. Thunderbird Medication and Vaccination Program. For every sabungeros and gamefowl fanatics out there. FREE SHIPPING on all supply order totals exceeding $25.00. On this page you will find chicken medications for better health and treatments for chicken diseases. We offer several different types of chicken vitamins/electrolytes and products for sale for treatment of parasites or worms in chickens.
- Vaccination plays a very big role in disease protection for any kind of poultry breeds and from this reason, Neochicks poultry limited offers free consultancy regarding the vaccination program by offering a comprehensive improved kienyeji chicken vaccination schedule.
- RECOMMENDED MEDICATION AND VACCINATION PROGRAMS FOR GAME FOWLS AGE(da y) 1 1-3 DRUGS INDICATION DOSAGE SC injection 1ml/10mlof water first 2 hrs. Scribd is the world's largest social reading and publishing site.
Abstract
Infectious laryngotracheitis (ILT) is an important respiratory disease of chickens and annually causes significant economic losses in the poultry industry world-wide. ILT virus (ILTV) belongs to alphaherpesvirinae and the Gallid herpesvirus 1 species. The transmission of ILTV is via respiratory and ocular routes. Clinical and post-mortem signs of ILT can be separated into two forms according to its virulence. The characteristic of the severe form is bloody mucus in the trachea with high mortality. The mild form causes nasal discharge, conjunctivitis, and reduced weight gain and egg production. Conventional polymerase chain reaction (PCR), nested PCR, real-time PCR, and loop-mediated isothermal amplification were developed to detect ILTV samples from natural or experimentally infected birds. The PCR combined with restriction fragment length polymorphism (RFLP) can separate ILTVs into several genetic groups. These groups can separate vaccine from wild type field viruses. Vaccination is a common method to prevent ILT. However, field isolates and vaccine viruses can establish latent infected carriers. According to PCR-RFLP results, virulent field ILTVs can be derived from modified-live vaccines. Therefore, modified-live vaccine reversion provides a source for ILT outbreaks on chicken farms. Two recently licensed commercial recombinant ILT vaccines are also in use. Other recombinant and gene-deficient vaccine candidates are in the developmental stages. They offer additional hope for the control of this disease. However, in ILT endemic regions, improved biosecurity and management practices are critical for improved ILT control.
GENERAL BIOLOGY OF INFECTIOUS LARYNGOTRACHEITIS VIRUS
Infectious laryngotracheitis (ILT) is a member of the Herpesviridae, the genus Iltovirus and Gallid herpesvirus 1 species[-]. It was first reported in the 1920s; however, this disease may have existed in chickens much earlier[4]. The genome of ILT virus (ILTV) is linear and approximated 150 kb of double-stranded DNA. It consists of long and short unique regions (UL, US) and two inverted repeat sequences (internal repeat; terminal repeat) that flank the US regions. An assembled complete genome sequence of ILTV from different strains contains 148 665 base pairs, and a G + C content of 48.16%. It was predicted that the genome had 77 open reading frames (ORFs), and 63 of these were homologous to herpes simplex virus-1 genes[-]. Most recently, 2 research groups used robust high-throughput methods to sequence the full length of vaccine and virulent ILTVs. The Australian group sequenced the complete genome of 3 chicken-embryo origin (CEO) vaccines, which were European strain, Serva, and 2 Australian strains, SA2 and A20. The genomes of the three CEO vaccines had 99% identity, and few single nucleotide polymorphisms were identified. However, the virulence was different between SA2 andA20 ILTVs[,]. An American research group sequenced the complete sequences of 4 virulent strains from different genotypes, and compared them with the Serva strain and the composite sequences. The vaccine and virulent ILTVs were 150, 335 to 153, 633 base pairs with 80 predicted ORFs. They are highly conserved and only 4 ORFs were different in length and 13 to 49 amino acids changed[]. These full genomic sequences contributed to further knowledge of the viral pathogenic factors, gene functions, and vaccine development. Although ILTV, Marek’s disease virus, and herpesvirus of turkey (HVT) belong to Alphaherpesvirinae, neither the nucleotide sequence nor the deduced amino acid sequence of glycoprotein D and ICP27 were similar. According to phylogenic analysis, ILTV could be an early type of α-herpesviruses[,].
ILTV is an enveloped virus and sensitive to heat, ether, chloroform, and other lipolytic solvents[]. Different strains of ILTV have different resistance to heat. At lower temperatures, ILTV maintains infectivity for a long period. The virus survived for days to months at 13-23 °C in tracheal exudates and chicken carcasses[12]. When stored at -20 °C to -60 °C, ILTV was viable for months to years. Storage media containing glycerol or sterile skim milk greatly increases the infectivity in tracheal swabs[,14]. It has been shown that the virus was destroyed in 1 min by treated with 3% cresol or a 1% lye solution[]. On a chicken farm, 5% hydrogen peroxide mist administered with fumigation equipment completely inactivated ILTV[].
Hosts of ILTV
All ages of chickens are affected, but chickens older than 3 wk are most susceptible to ILTV[]. It has been shown that ILTV can infect pheasants, pheasant-bantam crosses, and peafowl[]. ILT can infect turkeys at about 100 d of age. Clinical signs of dyspnea and depression can be observed in infected turkeys[]. Other avian species are resistant to ILTV infection[,20]. Embryonating chicken eggs are the most common method for propagating ILTVs. In chicken embryos, ILTV forms plaques on the chorioallantoic membrane (CAM). The plaques can be observed 48 h after infection, and embryos can die in 2-12 d post infection (PI). Strains of ILTV showed different plaque size and morphology on the CAM[21-]. The ILTV can be isolated in primary cell cultures, such as chicken embryo liver (CEL), chicken embryo kidney (CEK), and chicken kidney (CK) cell cultures. The sensitivity of ILTV isolation and propagation from field samples vary depending on the type of cell cultures. CEL was the most sensitive for isolation, followed by CK. The CEK and chicken embryo lung cells were less sensitive. Chicken embryo fibroblasts, Vero cells, and quail cells were not satisfactory for primary isolation of ILTV. Lymphocytes, thymocytes, and activated T cells were not sensitive to ILTV infection[-].
Transmission and latent infection: Natural transmission of ILTV is through the upper respiratory and ocular routes. Sources of ILTV are clinically affected chickens, latent infected carriers, contaminated dust, litter, beetles, drinking water and fomites[,27,28]. Our recent study showed that ILTV can remain in biofilm of drinking water lines and subsequently be transmitted to susceptible birds. Biofilm is a sticky substance produced by bacteria, which can render microorganism resistant to some routinely used sanitizers. Commercial sodium hydrogen sulfate (pH water treatment, PWT®, Jones-Hamilton Co., Walbridge, OH) and hydrogen peroxide (Proxy-Clean®, Kanters Special Products USA, St. Paul, MN) were able to inactivate ILTV in the water lines[27] (Table (Table1).1). Darkling beetles in the chicken farms are possible sources to transmit ILTV. Our investigation revealed that in ILTV infected chicken houses, the darkling beetles contained live virus at least 42 d after the disease outbreak[28] (Table (Table2).2). Other possible sources of transmission included dog, crows, and cats[29]. Wind-borne transmission between farms was critical for ILTV spread[30]. The tissue culture origin (TCO) and CEO vaccines served as a model for ILTV transmission as well as replication via eye drop route. The viruses replicated mainly in the conjunctiva and trachea. The vaccine viruses can be re-isolated and viral DNA can be detected from contact exposed birds as early as 7 d after exposure[].
Table 1
Viral isolation from swabs in specific-pathogen-free embryonating eggs after sanitizer treatments
| Sanitizer | Sample | 1st d | 7th d | 14th d | 21st d |
| Sodium hypochlorite | Trachea | -1 | - | +2 | + |
| Drinker | - | - | + | + | |
| Biofilm | ND | ND | ND | + | |
| Citric acid | Trachea | - | - | + | + |
| Drinker | - | + | + | + | |
| Biofilm | ND | ND | ND | + | |
| Sodium hydrogen sulfate | Trachea | - | - | - | - |
| Drinker | - | - | - | - | |
| Biofilm | - | - | - | - | |
| Hydrogen peroxide | Trachea | - | - | - | - |
| Drinker | - | - | - | - | |
| Biofilm | - | - | - | - | |
| Positive control3 | Trachea | - | - | + | + |
| Drinker | - | + | + | + | |
| Biofilm | + | + | + | + | |
| Negative control4 | Trachea | - | - | - | - |
| Drinker | - | - | - | - | |
| Biofilm | - | - | - | - |
Table 2
Infectious laryngotracheitis virus isolation from beetles in specific-pathogen-free embryos
| Days after outbreak | Before H2O2 treated | After H2O2 treated | |
| Farm 1 | 17 | -1 | - |
| 53 | - | - | |
| 103 | - | - | |
| Farm 2 | 13 | 2 | + |
| 42 | + | - | |
| 90 | - | - |
Farm 1: Negative for virus isolation; Farm 2: Positive for virus isolation.
ILTV can persist in the infected birds. The virus can be re-isolated from tracheal swabs 7 wk PI, or 2 mo PI in tracheal samples. The trigeminal ganglion is the target for ILTV latency. Fifteen months after vaccination, ILTV in the trigeminal ganglion was reactivated. In mature laying chickens challenged with virulent ILTV, DNA was detected in the trigeminal ganglion by polymerase chain reaction (PCR). The ILTV DNA can be detected in the trigeminal ganglion at 2 d after birds were vaccinated via eye drop route. When birds were stressed, such as the onset of lay or re-housing, ILTV was re-activated and spread to susceptible birds[-].
Clinical signs and lesions: There are two clinical forms of ILT infection (severe and mild). Clinical signs of the severe form include dyspnea and bloody mucus. This form can cause high morbidity and mortality up to 70%[,34]. The mild form includes depression, reduced egg production and weight gain, conjunctivitis, swelling of the infraorbital sinuses (almond shaped eyes), and nasal discharge. ILT takes 10 to 14 d for recovery, but with some strains the clinical signs may extend for weeks[35]. The mild form is the most commonly seen type in the US and is called “silent, vaccinal, or almond-shape eye” ILT.
Gross lesions are observed in the larynx and trachea. With the severe form, the mucosa of the respiratory tract shows inflammation and necrosis with hemorrhage. A characteristic feature is intranuclear inclusion bodies in epithelial cells. Inclusion bodies are generally present for a few days at the early stage of infection before epithelial cells die. Epithelial cells also form multinucleated cells (syncytia). When the necrotic epithelial cells detached from the trachea, bloody mucus was observed[35,36].
Detection and identification of ILTV
Laboratory diagnosis is required for ILT, because other diseases cause similar clinical signs and lesions, such as infectious bronchitis, Newcastle disease, avian influenza, infectious coryza, and mycoplasmosis. ILTV infection can be confirmed using several methods, including virus isolation and DNA detection. A commercial ELISA for detection of antibodies against ILTV is available; however, the test is not used for routine laboratory diagnosis. For ILTV isolation, the CAM inoculation of 9-to-12-d-old embryos and primary cell culture are used. Swab or organ samples from the trachea, conjunctiva, larynx, and lung of clinically affected birds are collected and inoculated on the CAM. The CEL and CK cell cultures were suitable for ILTV isolation. Multinucleated giant cells may be observed 24 h PI[,35].
Traditional antigen detection uses ILTV polyclonal or monoclonal antibodies to bind ILTV antigen from clinical samples. Viral antigen was detected using direct or indirect fluorescent antibodies (FA) in the tracheal smear or tracheal tissues[]. A more sensitive method using immunoperoxidase (IP) labeled monoclonal antibodies can be used as immunoprobes to detect ILTV in tracheal smears. This IP method detected ILTV on the second day PI[]. Agar gel immunodiffusion uses hyperimmune serum against ILTV to detect antigen in tracheal samples and it can differentiate ILT from the diphtheritic form of fowl pox. However, the sensitivity was lower than with other methods[39]. Antigen capture enzyme-linked immunosorbent assay (AC-ELISA) uses ILTV monoclonal antibodies for antigen detection. The AC-ELISA was faster and more accurate than AGIP or FA[].
ILT DNA detection methods have developed rapidly in recent years. These methods can identify ILTV quickly, accurately, and are highly sensitive. Molecular techniques for ILTV detection include cloned DNA probes for dot-blot hybridization, PCR, nested PCR, real-time PCR, multiplex PCR, in situ hybridization[-], and PCR followed by restriction fragment length polymorphism (RFLP)[,-]. ILTV detection with PCR was more sensitive than virus isolation in cell culture and electron microscopy. PCR also detected ILTV in the samples, which were contaminated with other pathogens[]. Compared with electron microscopy, histologic test, viral isolation via CAM route, FA test, and real-time PCR for ILT diagnosis in a broiler farm outbreak, the real-time PCR was the most sensitive method for ILTV detection[]. Since many laboratories are not set up for real-time PCR; FA, histopathology, and PCR are the most commonly used methods used for routine diagnosis of ILT[].
Recently, we developed a novel nucleic acid detection method, loop-mediated isothermal amplification (LAMP), for ILTV DNA identification and compared the sensitivity and specificity with real-time PCR. Both methods are highly specific and sensitive. The LAMP assay can detect ILTV DNA at the concentration of 60 copies/μL in 45 min without expensive equipment and reagents. The real-time PCR has a detection limit at 10 copies/μL[]. The LAMP assay is suitable for basic diagnostic laboratory detection in the field and real-time PCR can be used for further verification.
ILTV strain differentiation
It is not possible to identify different strains of ILTV by serological methods, because ILTVs have close immunodominant domains[56]. The most common and effective molecular method for ILTV differentiation is PCR followed by RFLP. PCR-RFLP analysis of single or multiple viral genome regions can differentiate strains from various geographic areas and vaccine from field strains[,-]. Restriction endonuclease analysis of ILTV DNA can differentiate vaccine strains from wild type strains[]. Moreover, PCR-RFLP analysis of the partial ICP4 gene, gC gene, and TK gene can distinguish field strains from vaccines. However, some virulent isolates could not be separated from vaccine strains[]. Han et al[] (2001) analyzed multiple genes with PCR-RFLP combined with DNA sequence analysis of the gG and TK genes to differentiate vaccine and non-vaccine strains. Researchers demonstrated that multiple gene PCR-RFLP was more reliable to differentiate vaccines from field strains[]. A new reverse RFLP method was reported to separate vaccine from non-vaccine ILTV strains. This method combined real-time quantitative PCR and restriction enzyme digestion and calculated the change of cycle threshold number value between digested and undigested template DNA for examining the genotype of ILTVs[].
Oldoni et al[] (2007) investigated ILTV isolates from commercial poultry that were collected between 1988 and 2005 using multiple gene PCR-RFLP analysis (ORFB-TK, ICP4, UL47/gG, and gM/UL9). They were able to separate ILTVs into nine genetic groups. Group I and II comprised the USDA reference strain and TCO vaccine strains. Group IV isolates were identical to CEO vaccine strains, whereas group V isolates, which had one PCR-RFLP pattern different from the CEO vaccine strains were CEO-related isolates. Group VI, VII, VIII, and IX were field ILTV strains with genomic types different from CEO and TCO vaccines. These groups also showed different isolates based on pathogenicity. In that report, most of ILTV positive poultry isolates were related to vaccine strains[]. Oldoni et al[] in 2008 investigated 46 ILTV field isolates collected in the US from 2006 to 2007. After multiple PCR-RFLP genotype analysis, many isolates were similar to vaccine strains[]. According to these reports, most ILTV field isolates in the US might come from vaccine reversion. Differentiation of vaccine from field viruses is important since countries can initiate trade barrier for importation of chicken products from areas where virulent field viruses exist. Therefore, the more common mild ILT is commonly referred to a “vaccinal” ILT even though PCR-RFLP testing has not always been done.
In Europe, 104 field isolates were collected during 35 years from eight different countries. These virus isolates were analyzed with PCR-RFLP targeting the TK gene and it was shown that they separate into 3 genetic groups. It was also shown that 98 of these field isolates had the same RFLP patterns as vaccine strains[]. In Australia, PCR-RFLP was used to analyze ILTV gG, TK, ICP4, ICP18.5 and ORFB-TK genes in 20 strains. These isolates could be discriminated into five genetic groups. Some isolates were closely related to vaccine strains[].
IMMUNITY AND VACCINATION
ILT vaccine induces protection against challenge in 1 wk. Humoral immunity is not the major immune response against ILTV in chickens. Research verified the importance of cell-mediated immunity (CMI) in the resistance to ILTV. An experiment was designed in which chickens were bursectomized with cyclophsophamide and surgical methods to block the humoral immune responses. Vaccinated bursectomized chickens developed protective CMI responses against virulent ILTV challenges[]. It confirmed that CMI was more important than humoral immunity. Furthermore, local CMI responses in the trachea produced protection from ILTV challenge in bursectomized chickens. Mucosal antibodies were not essential for resistance to challenge[].
Vaccination is effective in the prevent ILTV infection. However, ILT vaccine viruses can create latent infected carrier chickens. These latent carriers are a source for spread of virus to non-vaccinated flocks. Therefore, it is recommended that ILT vaccines be used only in endemic areas. The most currently used ILT vaccine strains are attenuated modified-live TCOs or CEOs viruses. Compared with protection afforded by TCO and CEO vaccines, there was no significant difference in the immunity of chickens at 10 wk post vaccination. However, when chickens over 20 wk of age were vaccinated, the CEO vaccines induced better protection than TCO vaccines[]. Methods for live vaccine administration are eye drop, drinking water, and aerosol spray. The drinking water route poses some problems in that chickens might not receive enough viruses at the target organ (nasal epithelial cells) and drinking water quality varies between poultry houses. Thus, these birds may fail to develop protective immunity and may have rolling (continual) reactions[]. On the other hand, with spray route, some chickens may develop severe reactions, because excess dosage of small droplets can penetrate deep into the respiratory tract[].
Reports have shown that modified-live vaccines increase their virulence by bird-to-bird passage. Serially passaged modified-live ILT vaccines in vivo for 35 generations. After the 6th passage, this vaccine strain produced severe clinical signs in challenged chickens. Furthermore, restriction endonuclease analysis of the viral genomes between original and final passage showed no differences between isolates. CEO vaccines have the tendency to increase in virulence more than TCO vaccines, when passed in chickens[,]. Investigations of ILTV isolates collected from around the world were analyzed by PCR-RFLP. They revealed that some current field virulent isolates were closely related to vaccine strains. This implies that field isolates originated from vaccine strains after back passage in chickens[,-].
Recently recombinant vaccines have been commercialized. Including partial ILTV genes were inserted into fowlpox and HVT modified genomes. A recombinant fowlpox vaccine, which contained ILTV glycoprotein B (gB) gene, was shown to induce protection against virulent strains[]. Another recombinant fowlpox virus, which contains ILTV gB and UL 32 genes, showed some efficacy to provide protection against virulent strain challenge via wing web administration[]. Two licensed commercial recombinant ILT vaccines are used in the US. One is produced by the CEVA (Biomune Company, Lenexa KS), which uses fowl poxvirus as a vector with an insertion of ILTV gB and UL 32 genes. The other is produced by Intervet (Intervet, Inc. Millsboro, DE), in which ILTV gI and gD genes are cloned into HVT. When these licensed commercial recombinant ILT vaccines were vaccinated by 18-d-old embryos in ovo injection, they reduced the clinical signs, but not virus replication after challenge[]. These recombinant ILTV vaccines did not cause latent infections and virulent reversion. Although these recombinant vaccines are safer than previously developed live vaccines, their increased cost and the fact that they must be injected has limited their use.
Several studies tried to develop new ILT vaccine candidates by gene deletion. Some ILTVs, with deleted virulent viral genes, retained their ability to induce immune responses without producing clinical signs and latency. Recombinant virus with deleted gJ, TK, and, UL0 genes readily showed attenuation, and could be used for vaccine production[-]. The gG-deficient ILTV, administered by either eye drop or drinking water routes for 3-wk-old specific-pathogen-free birds, induce adequate immunity against challenge. Therefore, it may be able to a use for large-scale vaccination. However, further studies need to be done to determine the protection of this gG-deficient vaccine on commercial chicken farms[]. There were also ILTV non-essential genes, which were deleted to test their ability as vaccines. The ILT mutants, which had five unique ORF A-E deleted, removed gN and gM, and the green fluorescent protein was inserted into the UL50 gene deleted region[-]. The gC deleted ILTV resulted in reduced virulence and it could be a marker vaccine[]. These recombinant and gene-deleted ILTVs could be used as candidates to differentiate vaccinated from field-infected birds. Recombinant vaccines used ILTV as a viral vector to contain H5 or H7 genes of highly pathogenic AIVs. These recombinant ILTV may protect birds from ILT and pathogenic AIV[,]. Recently, a new ILTV vector had a HPAI H5 gene inserted into a deleted UL50 gene region. This recombinant virus protected birds from homologous and heterologous H5N1 and H5N2 viruses challenge[,]. An ILTV gB gene DNA vaccine was developed. The gB gene combined with chicken IL-18 as bicistronic vector induced better protection in chicken from ILTV challenge than the gB gene monocistronic vector alone[]. Using ILTV gB gene plasmid DNA vaccine and chicken IL-18 plasmid DNA as an adjuvant induced T helper-1 immune response, which protected birds from virulent ILTV challenge[].
Prevention and control of ILTV infection using chicken house management
It is important to avoid contact between vaccinated or recovered field virus infected birds with non-vaccinated chickens. It is also critical to remove contaminated fomites for prevention and control of ILTV infection. To control ILTV outbreaks, improved biosecurity and management practices are necessary. Biosecurity includes protocols and procedures to prevent pathogens from infecting and transmitting disease by humans, insects, wild birds, or other animals[87]. A study found that heating litter at 38 °C for 24 h, using commercial litter treatments, and in-house composting for 5 d reduced this virus below detection levels[88,89]. Rapid diagnosis, a suitable vaccination procedure, and co-operation between government and industry are critical for ILT control. In 2005, California had a “vaccinal LT” outbreak in broiler farms. Although the companies improved the biosecurity and vaccination in these farms, they did not stop the chickens from being infected with ILT. Therefore, a strategy of depopulation, extended downtime, and strict biosecurity eliminated ILT in these farms was performed[]. Recently, for controlling the outbreaks, geographic information systems were used to provide the information of the regions for biosecurity, quarantine, vaccination, and the route to processing plants. Government agents, industry companies, growers, and veterinarians need to work together and design a program for outbreak control[].
CONCLUSION
ILT continues as an economical important poultry disease. House management and biosecurity measures should be performed for disease control. For eradication ILT, the modified-live vaccines need to be replaced by improved recombinant vaccines for the prevention of latent infection and virulent reversion.
Footnotes
Peer reviewer: Jean-Michel Garcia, PhD, HKU-Pasteur Research Centre, Translational Research group Leader, Dexter H.C. Man Building, 8, Sassoon Road, Pokfulam, Hong Kong, China
S- Editor Wang JL L- Editor A E- Editor Zheng XM
References

Gamefowl Eggs And Chicks For Sale
Vaccination Program For Gamefowl Chicks For Sale
Gamefowl Chicks For Sale Oklahoma
Vaccination Program For Gamefowl In Philippines
Gamefowl Roosters For Sale Chicks
Gamefowl Vaccine - Gamefowl Vaccine Protection by Agribusiness Philippines
Know the importance of the onset protection and how to determine this. Learn more about Gamefowl vaccine as protection of Live and Killed Vaccine are explained. Discover the advantage of administering Killed Vaccine.
Agribusiness How It Works Philippines. Agriculture and Agribusiness opportunities for the Overseas Filipino Worker (OFW) and their families. Instruct. Inspire. Succeed.
Agribusiness - How It Works, Sundays between 8:00AM to 9:00AM
ABS-CBN Sports + Action Bee Farming Success Story : Milea Bee Farming | Agribusiness Philippines. Livewire Complete Vaccination. Cattle Farming Part 1 : Cattle Farming in the Philippines | Agribusiness Philippines. Pekin Ducks Farming : Pekin duck Ducklings Supplement | Agribusiness Philippines. Sabong Vet (Vitamin Deficiency). Peking Duck Farming Success Story : Ang Tindahan ng Itlog ni Kuya | Agribusiness Philippines. Joey Lapid interview Part1 Gamefowl Conditioning and Pointing from the Expert. Why Vaccination is a MUST. Iron and B Complex Supplements. Take a 360° Virtual Reality Tour of a Chicken Hatchery.